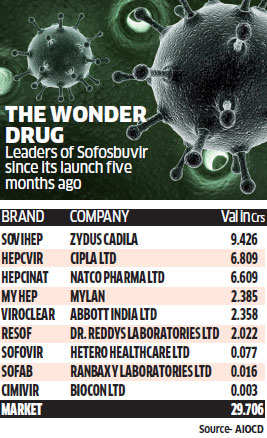
For patients with Hepatitis C, Dr Parveen Malhotra prescribes a tablet that doctors say is revolutionising the treatment paradigm for the dreaded liver ailment. The hepatologist from Haryana's Rohtak town too has reported a higher cure rate since switching to the orally administered sofosbuvir from the injectable interferon five months ago.
According to World Health Organization data, hepatitis C kills half a million people a year and infects 150 million globally. Screening often includes costly multiple tests without which the ailment often goes undetected. In this backdrop, say doctors, sofosbuvir, is proving to be a magic bullet, unlike some of the alternatives that came with a host of side effects.
"This molecule (sofosbuvir) is revolutionary. Earlier we used to treat with interferon therapy, but here you have for the first time a therapy in oral form. With this molecule the ease of treatment has improved," said Dr Mandar Kubal, director of Mumbai based Infectious Diseases & Pulmonary Care.
Read more...
Welcome to HCV Advocate’s hepatitis blog. The intent of this blog is to keep our website audience up-to-date on information about hepatitis and to answer some of our web site and training audience questions. People are encouraged to submit questions and post comments.
For more information on how to use this blog, the HCV drug pipeline, and for more information on HCV clinical trials click here
Be sure to check out our other blogs: The HBV Advocate Blog and Hepatitis & Tattoos.
Alan Franciscus
Editor-in-Chief
HCV Advocate
Showing posts with label India. Show all posts
Showing posts with label India. Show all posts
Friday, September 11, 2015
Monday, May 4, 2015
India: Mylan launches hepatitis-C Sovaldi tablets in India
HYDERABAD: Pharma giant Mylan NV today said its subsidiary Mylan Pharmaceuticals has launched Gilead Sciences' Sovaldi (sofosbuvir 400mg tablets) in the country.
Sovaldi is used for the treatment of chronic hepatitis-C infection as a component of a combination anti-viral treatment.
It is estimated that around 12 million people are chronically infected with hepatitis-C in India, Mylan said in a release.
In February this year, Gilead appointed Mylan as its exclusive distributor of Sovaldi in India.
Read more at: http://economictimes.indiatimes.com/articleshow/47148108.cms?utm_source=contentofinterest&utm_medium=text&utm_campaign=cppst
Sovaldi is used for the treatment of chronic hepatitis-C infection as a component of a combination anti-viral treatment.
It is estimated that around 12 million people are chronically infected with hepatitis-C in India, Mylan said in a release.
In February this year, Gilead appointed Mylan as its exclusive distributor of Sovaldi in India.
Read more at: http://economictimes.indiatimes.com/articleshow/47148108.cms?utm_source=contentofinterest&utm_medium=text&utm_campaign=cppst
Friday, April 24, 2015
India: Mylan Pharmaceuticals launches generic hepatitis C drug MyHep in India
NEW DELHI: Drugmaker Mylan Pharmaceuticals today launched generic Sofosbuvir tablets, indicated for the treatment of chronic hepatitis C, in the country.
US-based company's Indian arm Mylan NV has launched generic Sofosbuvir tablets in strength of 400 mg under the name MyHep in the country, it said in a statement.
"The launch of Mylan's MyHep offers hope to millions of hepatitis C patients in India who are in need of a high quality, effective and affordable treatment option."
US-based company's Indian arm Mylan NV has launched generic Sofosbuvir tablets in strength of 400 mg under the name MyHep in the country, it said in a statement.
"The launch of Mylan's MyHep offers hope to millions of hepatitis C patients in India who are in need of a high quality, effective and affordable treatment option."
Labels:
generic Sovaldi,
India,
MyHep,
Mylan
Friday, March 27, 2015
India: Strides Arcolab Launches Generic Version of Hepatitis C Drug
New Delhi: Strides Arcolab on announced launch of the generic version of Hepatitis C drug 'Sofosbuvir' under the brand name 'Virso'.
The product will be available to Indian patients shortly, the Bangalore-based company said in a statement.
"In September 2014, Strides entered into a licensing agreement with Gilead Sciences Inc to bring Hepatitis C cure to 91 developing countries," Strides Arcolab said in a statement.
Read more...
The product will be available to Indian patients shortly, the Bangalore-based company said in a statement.
"In September 2014, Strides entered into a licensing agreement with Gilead Sciences Inc to bring Hepatitis C cure to 91 developing countries," Strides Arcolab said in a statement.
Read more...
Labels:
generic Sovaldi,
India,
Virso
Wednesday, March 25, 2015
Cipla launches generic Hepatitis C drug Hepcvir
Cipla, on Wednesday, announced the launch of generic drug Sofosbuvir for treating chronic Hepatitis C under the brand name Hepcvir.
“Following the non-exclusive licensing agreement signed with Gilead Sciences in September last to manufacture and market chronic Hepatitis C medicines, Cipla is now all set to make the drug Sofosbuvir available to Indian patients in a week’s time,” the company said in a statement.
Monday, March 23, 2015
India: Portal to create awareness on Hepatitis C launched
Hyderabad, March 23: Asian Institute of Gastroenterology (AIG) has launched a portal to create awareness on Hepatitis C.
This was formally launched by E S L Narasimhan, Governor, Andhra Pradesh and Telangana, at a function here on Monday.
Speaking on the occasion, D Nageshwar Reddy, Chairman of AIG, said out of an estimated 150 million people affected by Hepatitis C in the world, 15 million were from India.
See also: Most Hepatitis C affected untreated in India
See also: Most Hepatitis C affected untreated in India
Labels:
Awareness,
Epidemiology,
India
India: Dr Reddy's to launch Hepatitis C drug in India
NEW DELHI: Dr Reddy's Laboratories today entered into an agreement with Hetero to distribute and market generic version of US-firm Gilead Sciences' Hepatitis C drug under the brand 'Resof' in India.
"The company has entered into an agreement with Hetero, under which Dr Reddy's has been licensed to distribute and market Sofosbuvir 400 mg tablets indicated in treatment of chronic Hepatitis C under the brand 'Resof' in India," the Hyderabad-based drug major said in a BSE filing.
"The company has entered into an agreement with Hetero, under which Dr Reddy's has been licensed to distribute and market Sofosbuvir 400 mg tablets indicated in treatment of chronic Hepatitis C under the brand 'Resof' in India," the Hyderabad-based drug major said in a BSE filing.
Wednesday, March 18, 2015
India, Pharmacy to the Developing World, Must Honor IP Rights
The United States and India are locked in a vitriolic debate over intellectual property rights in the pharmaceutical sector. The tension between pharmaceutical patents and access to affordable medicines took center stage during President Obama’s three-day visit to India in January. For several years the United States has been increasing the pressure on India to adopt intellectual property protections similar to those of the U.S. and the European Union, without avail. According to the 2015 U.S. Chamber of Commerce’s Intellectual Property Index, India ranks 29th among 30 nations in their protection for intellectual property rights. The report scores nations in several IP dimensions, out of a maximum of 30 points. India scored 7.23 points, only Thailand was ranked lower, while the U.S., the highest-ranked country, scored 28.53 points.[1]
Claiming to be the “Pharmacy to the Developing World”, India argues that their lax intellectual property rights regime is critical to their ability to provide low-cost, quality generic drugs. They are wrong on two counts. First, India needs to honor IP rights, because without effective intellectual property rights, new pharmaceuticals will not be developed and the “Pharmacy to the Developing World” won’t have anything to provide to the developing world, or to anyone. Second, given the quality crisis in the Indian pharmaceutical industry, they shouldn’t be the pharmacy to anyone.
In early January 2015, the Indian government rejected Gilead Sciences Inc’s patent application for its Hepatitis C drug Sovaldi. This comes on the heels of numerous other attacks on pharmaceutical patents. As of mid-2014, India had “denied, revoked or otherwise attacked” the patents of 15 of the approximate 45 patented medicines on the Indian market.[2] The result is a regime of protectionism that coddles Indian industry at the cost of U.S. jobs. The pharmaceutical industry is but one of many industries experiencing such treatment. While the United States has welcomed Indian firms, India has shunned innovative U.S. firms. As described in his Pre-Hearing Statement to the U.S. International Trade Commission, Rod Hunter notes that Indian pharmaceutical firms have enjoyed unfettered access to the sizeable U.S. market.
Read more...
Claiming to be the “Pharmacy to the Developing World”, India argues that their lax intellectual property rights regime is critical to their ability to provide low-cost, quality generic drugs. They are wrong on two counts. First, India needs to honor IP rights, because without effective intellectual property rights, new pharmaceuticals will not be developed and the “Pharmacy to the Developing World” won’t have anything to provide to the developing world, or to anyone. Second, given the quality crisis in the Indian pharmaceutical industry, they shouldn’t be the pharmacy to anyone.
In early January 2015, the Indian government rejected Gilead Sciences Inc’s patent application for its Hepatitis C drug Sovaldi. This comes on the heels of numerous other attacks on pharmaceutical patents. As of mid-2014, India had “denied, revoked or otherwise attacked” the patents of 15 of the approximate 45 patented medicines on the Indian market.[2] The result is a regime of protectionism that coddles Indian industry at the cost of U.S. jobs. The pharmaceutical industry is but one of many industries experiencing such treatment. While the United States has welcomed Indian firms, India has shunned innovative U.S. firms. As described in his Pre-Hearing Statement to the U.S. International Trade Commission, Rod Hunter notes that Indian pharmaceutical firms have enjoyed unfettered access to the sizeable U.S. market.
Read more...
Tuesday, March 10, 2015
India: Natco to launch Hepatitis C drug in India soon
Natco Pharma plans to launch the generic version of Sovaldi, the blockbuster drug used to treat chronic Hepatitis C in India soon. Sovaldi is made by U.S. pharma major Gilead Sciences, and Natco recently entered into a non-exclusive licensing agreement with Gilead to make and sell generic versions of Sovaldi in 91 developing countries.
The company, on Monday, launched the generic version of Sovaldi in Nepal under the brand Hepcinat. The product is priced at Rs.19,900 (US $316.68) per bottle of 28 tablets in Nepal, and covers the treatment duration of three months for a patient.
“We will be launching the drug in the next few weeks in India, and are awaiting approval from the Drugs Controller General of India (DCGI),” M. Adinarayana, Vice-President, Legal & Corporate Affairs, Natco Pharma, told this correspondent.
Labels:
generic Sovaldi,
HEPCINAT,
India
Monday, March 2, 2015
India: Natco Pharma ties up with Gilead on hepatitis C drugs
(Reuters) - Natco Pharma Ltd said on Monday it has agreed a deal with Gilead Sciences Inc to supply generic copies of the U.S. drugmaker's chronic hepatitis C medicines, including $1,000-a-pill drug Sovaldi, in 91 developing nations.
Natco, a mid-sized player in India's crowded pharmaceutical industry, is the latest generic drugmaker to team up with Gilead on Sovaldi, having previously attempted to block the U.S. firm from getting a patent for the breakthrough drug in India in the hope of producing a cheaper version on its own. In September, Gilead announced similar licensing deals with seven other generic drugmakers.
Read more...
Natco, a mid-sized player in India's crowded pharmaceutical industry, is the latest generic drugmaker to team up with Gilead on Sovaldi, having previously attempted to block the U.S. firm from getting a patent for the breakthrough drug in India in the hope of producing a cheaper version on its own. In September, Gilead announced similar licensing deals with seven other generic drugmakers.
Read more...
Wednesday, February 11, 2015
India: Cipla to name Gilead hepatitis C generic Hepcvir
Pharma major Cipla plans to name its hepatitis C drug with Gilead Sciences as Hepcvir, its chairman Yusuf K Hamied on Wednesday. Last year Gilead Sciences has licensed its hepatitis C drug Sovaldi (chemically known as sofosbuvir) to seven India-based drugmakers including Cipla which will sell the cheap generic versions of the drug in 91 developing nations. Hepcvir is the brand name and we are working on it. We will apply probably for regulatory approval in the next couple of months and hopefully by middle of the year we will be in market for India. We have the stocks of the raw materials, we are already started making the raw materials. We have to put the stability data of active pharmaceutical ingredients, stability data of the formulation, we have to do bioequivalence of the originator product, so these takes around six months and this is already in process," he said.
The Mumbai-based drug maker has signed a non-exclusive licensing agreement with Gilead for manufacturing and distribution of Sofosbuvir mono, Ledipasvir mono, the fixed-dose combination of Ledipasvir/Sofosbuvir with each other and the combination of Sofosbuvir or Ledipasvir with other active substances, for the treatment of hepatitis C.
All companies have been allowed to set their own prices for the generic drug and will pay Gilead a royalty on their sales.
Read more...
The Mumbai-based drug maker has signed a non-exclusive licensing agreement with Gilead for manufacturing and distribution of Sofosbuvir mono, Ledipasvir mono, the fixed-dose combination of Ledipasvir/Sofosbuvir with each other and the combination of Sofosbuvir or Ledipasvir with other active substances, for the treatment of hepatitis C.
All companies have been allowed to set their own prices for the generic drug and will pay Gilead a royalty on their sales.
Read more...
Saturday, January 31, 2015
Delhi HC sets aside order on Gilead’s Sovaldi patent
New Delhi: In a setback to generic drug makers, the Delhi high court on Friday set aside an order of the Deputy Controller of Patents and Designs rejecting a patent to US drug maker Gilead Pharmasset Llc for the hepatitis C drug Sovaldi.
The medicine costs $1,000 a pill and cures hepatitis C in 90% of cases when given for a 12-week course. India’s patent office had questioned the therapeutic efficacy under which the patent was claimed by Gilead and rejected its application using the controversial Section 3(d) of the Patents Act, which prevents evergreening of patents and provides that no new form of an existing substance shall be patented unless the new form is much more effective than the old one.
The Indian patent office, while rejecting the patent application, had maintained that minor changes in the molecule did not improve its efficacy. With the patent set aside, domestic generic drug manufacturers could make the same drug for as low as $1 a pill. “It was expected that the appeal would succeed because the process of reasoning in the controller’s order was really shoddy, without commenting on the merits of the conclusion,” said Shamnad Basheer, former professor at National University of Juridical Sciences, Kolkata, and founder of intellectual property blog SpicyIP.
Read more at: http://www.livemint.com/Politics/1l6EyoCRGd45A6oT0qpsBO/Delhi-high-court-sets-aside-order-on-Gileads-Sovaldi-patent.html?utm_source=copy
The medicine costs $1,000 a pill and cures hepatitis C in 90% of cases when given for a 12-week course. India’s patent office had questioned the therapeutic efficacy under which the patent was claimed by Gilead and rejected its application using the controversial Section 3(d) of the Patents Act, which prevents evergreening of patents and provides that no new form of an existing substance shall be patented unless the new form is much more effective than the old one.
The Indian patent office, while rejecting the patent application, had maintained that minor changes in the molecule did not improve its efficacy. With the patent set aside, domestic generic drug manufacturers could make the same drug for as low as $1 a pill. “It was expected that the appeal would succeed because the process of reasoning in the controller’s order was really shoddy, without commenting on the merits of the conclusion,” said Shamnad Basheer, former professor at National University of Juridical Sciences, Kolkata, and founder of intellectual property blog SpicyIP.
Read more at: http://www.livemint.com/Politics/1l6EyoCRGd45A6oT0qpsBO/Delhi-high-court-sets-aside-order-on-Gileads-Sovaldi-patent.html?utm_source=copy
Saturday, January 24, 2015
India: Docs get training in liver transplant in Delhi
JAIPUR: There is a ray of hope for the patients who need liver transplant. A team of 14 doctors and 15 nurses from SMS Medical College has returned from Delhi after being trained in liver transplant operation at a private hospital.
According to sources, till now there is no facility for liver transplant in the state. The patients have to go to other states like Delhi and Tamil Nadu for such operations. The Sawai Man Singh Hospital is taking it on a priority basis to introduce the facility as soon as possible in the hospital.
Dr Ashok Jhajharia, who was one of the members of team, said, "It was fruitful 15-day training of liver transplant in Delhi. It will help in introducing the facility of liver transplant in the state."
Read more...
According to sources, till now there is no facility for liver transplant in the state. The patients have to go to other states like Delhi and Tamil Nadu for such operations. The Sawai Man Singh Hospital is taking it on a priority basis to introduce the facility as soon as possible in the hospital.
Dr Ashok Jhajharia, who was one of the members of team, said, "It was fruitful 15-day training of liver transplant in Delhi. It will help in introducing the facility of liver transplant in the state."
Read more...
Saturday, January 17, 2015
Gilead to appeal India patent ruling on hepatitis C drug
(Reuters) - U.S.-based Gilead Sciences Inc will appeal the Indian patent office's rejection of its application for hepatitis C drug Sovaldi, a move that could allow local drugmakers to launch cheaper generic versions of the $1,000-a-pill medicine.
The rejection relates to the patent application covering the metabolites, or small molecules, of sofosbuvir, the chemical name of Sovaldi.
Indian drugmaker Natco Pharma and the Initiative for Medicines, Access & Knowledge (I-MAK) had opposed Gilead's application on the grounds that the drug is not inventive enough compared with a previous formulation.
Read more...
Saturday, January 10, 2015
India: HCV infection in hemodialysis patients raises concerns
AURANGABAD: Hepatitis C virus (HCV) infection which is an important pathogen causing liver disease is becoming a major public health problem, with an estimated 25% to 40% prevalence in India. Raising concern over the issue, experts said patients on multiple blood transfusions have a high risk for HCV due to the involvement of multiple routes of infections, especially poor blood screening of blood and low standard of dialysis procedures.
They were speaking at a workshop at Dattaji Bhale blood bank, organised by the Aurangabad Thalassemia Society to create awareness about thalassemia on Friday.
Mahendrasingh H Chauhan, medical director and in charge of the blood bank said that high prevalence of Hepatitis C virus (HCV) has been reported among dialysis patients throughout the world. Serious efforts need to be taken to investigate HCV in patients undergoing hemodialysis (HD) treatment who are at great risk to HCV infection.
Read more...
They were speaking at a workshop at Dattaji Bhale blood bank, organised by the Aurangabad Thalassemia Society to create awareness about thalassemia on Friday.
Mahendrasingh H Chauhan, medical director and in charge of the blood bank said that high prevalence of Hepatitis C virus (HCV) has been reported among dialysis patients throughout the world. Serious efforts need to be taken to investigate HCV in patients undergoing hemodialysis (HD) treatment who are at great risk to HCV infection.
Read more...
Subscribe to:
Comments (Atom)