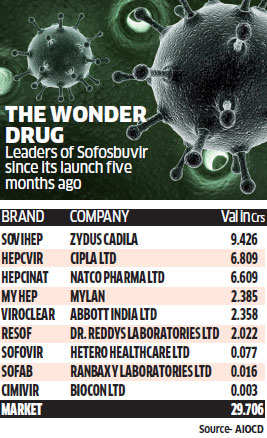
For patients with Hepatitis C, Dr Parveen Malhotra prescribes a tablet that doctors say is revolutionising the treatment paradigm for the dreaded liver ailment. The hepatologist from Haryana's Rohtak town too has reported a higher cure rate since switching to the orally administered sofosbuvir from the injectable interferon five months ago.
According to World Health Organization data, hepatitis C kills half a million people a year and infects 150 million globally. Screening often includes costly multiple tests without which the ailment often goes undetected. In this backdrop, say doctors, sofosbuvir, is proving to be a magic bullet, unlike some of the alternatives that came with a host of side effects.
"This molecule (sofosbuvir) is revolutionary. Earlier we used to treat with interferon therapy, but here you have for the first time a therapy in oral form. With this molecule the ease of treatment has improved," said Dr Mandar Kubal, director of Mumbai based Infectious Diseases & Pulmonary Care.
Read more...
Welcome to HCV Advocate’s hepatitis blog. The intent of this blog is to keep our website audience up-to-date on information about hepatitis and to answer some of our web site and training audience questions. People are encouraged to submit questions and post comments.
For more information on how to use this blog, the HCV drug pipeline, and for more information on HCV clinical trials click here
Be sure to check out our other blogs: The HBV Advocate Blog and Hepatitis & Tattoos.
Alan Franciscus
Editor-in-Chief
HCV Advocate
Showing posts with label generics. Show all posts
Showing posts with label generics. Show all posts
Friday, September 11, 2015
Friday, March 20, 2015
Pharma accused of restricting access to hep C drug in poor countries
New drugs for hepatitis C are a major breakthrough but hugely expensive in rich countries. Pharma giant Gilead will allow cheap copies to be made for poor countries - but only for patients with proof of identification and citizenship and the drug supplies will be closely tracked
The battle over access to the new hepatitis C drug, Gilead’s sofosbuvir (and similar drugs coming along behind) is hotting up. There is angst even in the richest countries over the $1000 a pill price tag. It now looks as though Gilead is going to extraordinary lengths to ensure that cheaper versions, which it is permitting generic companies to make for poor countries, do not arrive in affluent world pharmacies.
Gilead has agreed to grant voluntary licences to eleven Indian generic companies, which means the drug will be sold at a reduced price in low-income countries. But Médecins Sans Frontières, the volunteer doctors who are treating hepatitis C infection in some of the poorest regions, say the company has imposed unacceptable conditions.
Gilead stipulates that patients will only get the drug if they can provide identification, proof of citizenship and residency, which MSF says will penalise refugees and marginalised communities. The drug supplies will be closely tracked through codes on the bottles. Gilead, even though it will not be the manufacturer, will have access to that information. If they need more, patients will have to return an empty bottle.
Read more...
Subscribe to:
Comments (Atom)